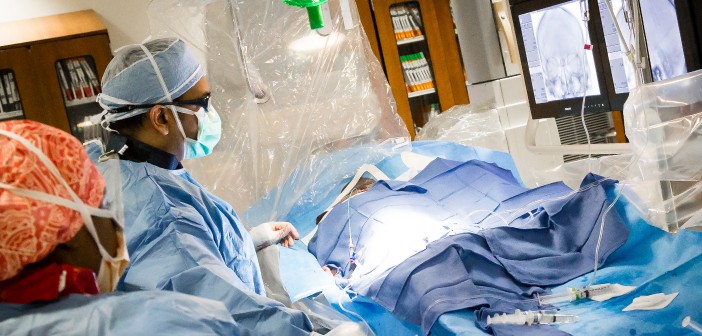
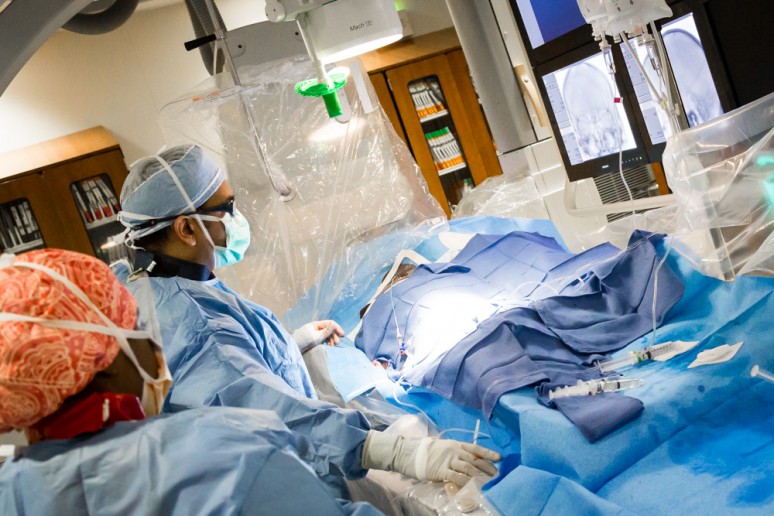
For many of us, the thought of undergoing surgery is terrifying – so, My City Wellness “scrubbed in” to get up close with a group of talented local physicians as they performed a variety of procedures. Our sincerest thanks to doctors Abed, Alkotob, Garg, Majjhoo and Munir for their gracious cooperation in creating this special section.
 Madar Abed, MD
Madar Abed, MD
PROCEDURE/SURGERY: Subcutaneous Implantable Cardiac Defibrillator (SICD)
CREDENTIALS: Director of EP Lab, Hurley Medical Center
FACILITY: Hurley Medical Center
METHOD/DESCRIPTION: To begin, the pulse generator (battery) is placed under the skin on the left side of the patient under the armpit/chest area. A high-voltage, L-shaped wire is put in, parallel to the sternum. There is a total of three incisions; the first is three inches (where the battery goes), and the other two are less than an inch at the top and bottom of the sternum (for the L-shaped lead). When the SICD detects an arrhythmia (V-FIB), it delivers a lifesaving shock to restore the heartbeat to sinus rhythm.
 The SICD procedure is less invasive than others; there are no leads in the blood vessels to the heart. In addition, there is less risk of stroke and complications, such as puncturing the heart or infections. The manufacturer, Boston Scientific, provides all the instruments and tools to implant the device more efficiently and with fewer complications.
The SICD procedure is less invasive than others; there are no leads in the blood vessels to the heart. In addition, there is less risk of stroke and complications, such as puncturing the heart or infections. The manufacturer, Boston Scientific, provides all the instruments and tools to implant the device more efficiently and with fewer complications.
Patients should be aware that the procedure will be done in the hospital, and they will be in a recovery room for 2-4 hours. The wound will have to be monitored and kept dry. If redness or swelling occurs, patients should contact their physician. There are no post-operative restrictions on driving or activity.
This is an outpatient procedure, and in most cases, patients will be released from the hospital the following day (24 hours). A full recovery should take place in about 4-6 weeks. By that time, patients will be back into their everyday lives.
 M. Luay Alkotob, MD
M. Luay Alkotob, MD
PROCEDURE/SURGERY: Percutaneous Coronary Intervention (PCI)
CREDENTIALS: Cardiology Hurley Medical Center, Board Certified Interventional Cardiologist, FACC, FSCAI
FACILITY: Hurley Medical Center
METHOD/DESCRIPTION: Through a catheter entering from the femoral (thigh) or radial (forearm) artery, a second catheter is inserted and engages the coronary artery in the heart. After identifying the narrowing location (blockage in the artery that supplies blood to the heart), a wire crosses the blockage to deliver a balloon that reduces the degree of narrowing and allows delivery of a metal mesh, called a stent. The stent is expanded to maintain patency (openness) of the artery and maximize blood supply to the heart muscle.
 Percutaneous Coronary Intervention is minimally invasive – no surgical cuts to the body. Compared to coronary artery bypass surgery, the recovery is very quick. Patients go home after an overnight stay for monitoring and may resume usual activities almost immediately.
Percutaneous Coronary Intervention is minimally invasive – no surgical cuts to the body. Compared to coronary artery bypass surgery, the recovery is very quick. Patients go home after an overnight stay for monitoring and may resume usual activities almost immediately.
The innovative, new-generation stents, the size of catheters and the availability of drug-eluting stents has resulted in higher efficacy and very low recurrence of blockages in the stents.
Having done this procedure over 1,000 times, Dr. Alkotob’s success rate is very high. Patients should be aware of surgeon’s experience level, as well as the risks, benefits and alternatives. With experienced operators, the risk of complications is very low (less than 1%). Patients should expect an overnight hospital stay, no heavy lifting for a week following surgery, and outpatient rehabilitation, both physical and cardiac.
 Gunjal Garg, MD
Gunjal Garg, MD
PROCEDURE/SURGERY: Radical Hysterectomy
CREDENTIALS: Dr. Garg treats all gynecologic cancers and has extensive training in minimally invasive surgery, robotic surgery, complex gynecologic procedures including extended pelvic resections, and pelvic reconstructive surgery. She is board certified in Obstetrics and Gynecology and board eligible in Gynecologic Oncology. Dr. Garg completed a residency in Obstetrics and Gynecology at Wayne State University/Detroit Medical Center and a fellowship in Gynecologic Oncology at Washington University in St. Louis, MO.
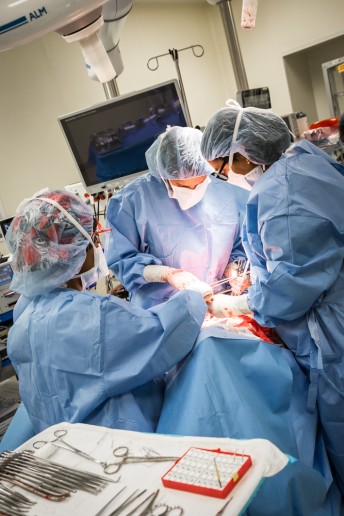
METHOD/DESCRIPTION: Once the incision is made, Dr. Garg begins by dissecting the spaces inside the pelvis to define the operating field. Next, the bladder is moved away from the uterus, cervix and upper vagina, and the uterine artery is tied off and cut at its origin from the internal iliac (or hypogastric) artery. The ureter is then completely dissected away from its surrounding tissue, allowing for a resection of the entire parametrial tissue, after which the rectovaginal space is opened by incising the peritoneum and the uterosacral ligaments are resected. Finally, the upper vagina is excised and the uterus, cervix, both parametria, the uretral sacral ligaments and upper vagina are all removed en bloc (together) through the vagina, after which the remaining vagina is sutured shut.
When a mass is confirmed to be cancerous, surgery may be scheduled with Dr. Garg directly. When a mass is detected on the reproductive organs but not confirmed cancerous, a gynecologist may request Dr. Garg to participate in surgery to ensure the most comprehensive care is given.
 Aniel Majjhoo, MD
Aniel Majjhoo, MD
PROCEDURE/SURGERY: Stent of a Cerebral Aneurysm
CREDENTIALS: Director of the McLaren Health Neuroscience Program and Stroke Network, Fellowship Trained in Endovascular Surgical Radiology/Interventional Neurology and Board Certified in Neurology, Vascular Neurology and Neurocritical Care. He has extensive training in all aspects of Neurology and has been the driving force behind the development of the McLaren Stroke Network.
FACILITY: McLaren-Flint
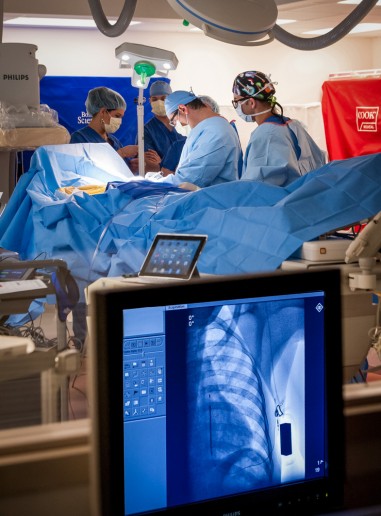
 METHOD/DESCRIPTION: The pipeline stent procedure allows the patient with an aneurysm to be treated in the least invasive method possible. With this procedure, there is no need to open the patient’s skull to close off an aneurysm, resulting in significantly less post-procedure complications, shorter hospital stays and quicker return to baseline function.
METHOD/DESCRIPTION: The pipeline stent procedure allows the patient with an aneurysm to be treated in the least invasive method possible. With this procedure, there is no need to open the patient’s skull to close off an aneurysm, resulting in significantly less post-procedure complications, shorter hospital stays and quicker return to baseline function.
The pipeline stent is the first and only FDA approved flow-diverting device for the treatment of brain aneurysms. Flow Diversion is a technique used to treat large or giant, wide-necked brain aneurysms in which the device is placed in the parent blood vessel rather than in the aneurysm sac. The Pipeline Device restores original, natural blood circulation while providing permanent, long-term occlusion.
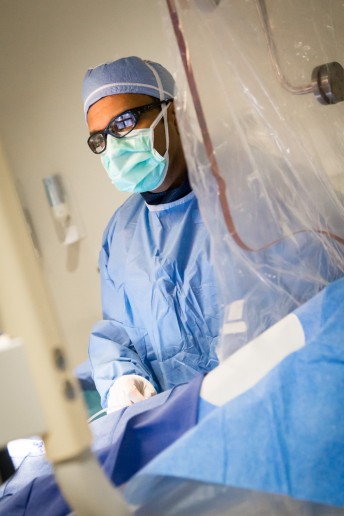 During the procedure, the Pipeline Device (a braided cylindrical mesh) is implanted across the aneurysm neck. This slows the flow of blood into the aneurysm, which allows for the diseased vessel to heal. Almost immediately, the blood flow to the aneurysm is reduced, and the complete closure of the aneurysm occurs between six weeks to six months after the procedure.
During the procedure, the Pipeline Device (a braided cylindrical mesh) is implanted across the aneurysm neck. This slows the flow of blood into the aneurysm, which allows for the diseased vessel to heal. Almost immediately, the blood flow to the aneurysm is reduced, and the complete closure of the aneurysm occurs between six weeks to six months after the procedure.
Patients will need to take a blood thinner (such as aspirin or Plavix) for at least six months after the procedure and follow up with the physician at three, six and 12 months post-procedure. The closure rate of aneurysms treated with the Pipeline Device is 96%, with 0% reoccurrence of aneurysm at five years post-procedure.
Most patients return to their baseline level of function within days of the procedure and resume normal activities within 2-4 weeks.
 Ahmad Munir, MD
Ahmad Munir, MD
PROCEDURE/SURGERY: TAVR (Transcatheter Aortic Valve Replacement), PFO (Patent Foramen Ovale)/ASD (Atrial Septic Defect) Closure, Mitral Valve Clip, Balloon Aortic and Mitral Valvuloplasty
CREDENTIALS: Dr. Munir earned his medical degree at King Edward Medical College in Lahore, Pakistan. He completed his residency at the University of Tennessee Health Science Center in Memphis. Dr. Munir continued training and completed his fellowship in Cardiovascular Diseases and Critical Care Medicine at the University of Tennessee Health Science Center where he was the Chief Fellow. He further completed his Interventional Cardiology fellowship at William Beaumont Hospital in Royal Oak, MI and Structural Heart Disease fellowship at Detroit Medical Center. Practicing cardiology for nearly 15 years, he is approaching his 400th TAVR case.
FACILITY: McLaren-Flint
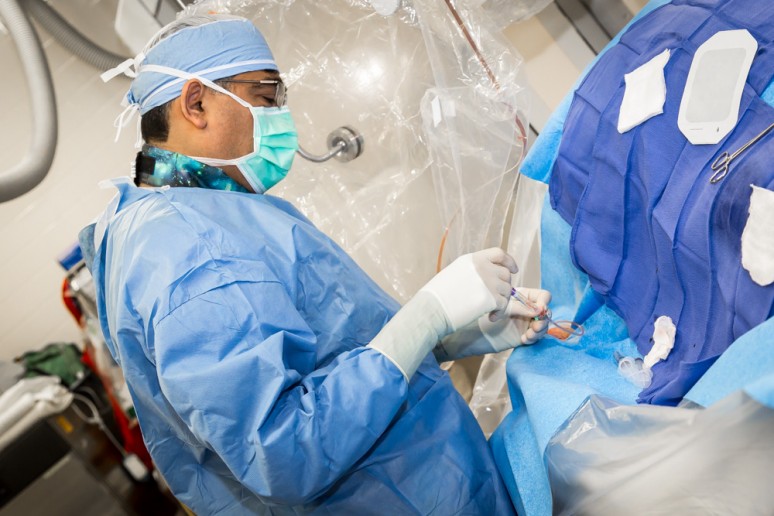 METHOD/DESCRIPTION: Structural heart diseases include cardiac defects that are related to heart valves and various chambers of the heart. These defects develop with wear and tear on the heart, through other disease processes or could be congenital in nature (birth defect). Advances in technology are allowing patients with these diseases to be treated with minimally-invasive procedures without open-heart surgery.
METHOD/DESCRIPTION: Structural heart diseases include cardiac defects that are related to heart valves and various chambers of the heart. These defects develop with wear and tear on the heart, through other disease processes or could be congenital in nature (birth defect). Advances in technology are allowing patients with these diseases to be treated with minimally-invasive procedures without open-heart surgery.
Transcatheter Aortic Valve Replacement (TAVR) is a revolutionary structural heart procedure developed for patients with aortic valve narrowing who are at high-risk for open-heart valve-replacement surgery. The FDA-approved procedure is performed in McLaren Flint’s state-of-the-art hybrid cath lab/operating room. In this lab, both minimally invasive and open-heart procedures can be performed in collaboration between cardiologists and cardiac surgeons.
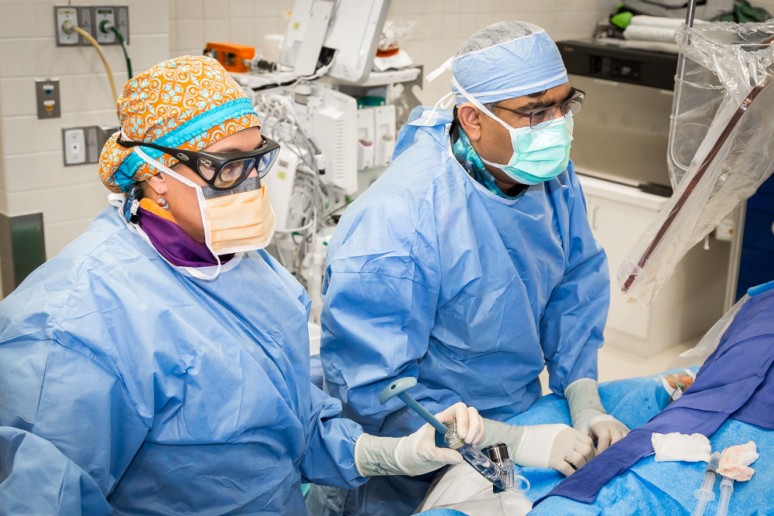 TAVR is performed by inserting an artificial valve via a delivery tube through small (few millimeter) incision in the groin. The recovery after TAVR is very fast due to the fact that no surgical incisions are made and the patient’s chest is not opened. Patients can go home in a few days and can resume their routine activities without much restriction.
TAVR is performed by inserting an artificial valve via a delivery tube through small (few millimeter) incision in the groin. The recovery after TAVR is very fast due to the fact that no surgical incisions are made and the patient’s chest is not opened. Patients can go home in a few days and can resume their routine activities without much restriction.
Patients being considered for the TAVR procedure undergo several diagnostic tests for eligibility. The patient is evaluated by a heart valve team, and the patient’s clinical status is discussed. Test results are then reviewed and risk for surgical and transcatheter valve replacement is evaluated. The options are discussed with the patient’s primary physician and cardiologist before a recommendation is made. The success rate with this procedure is greater than 95 percent.
Compiled by Sherron Barden and Allison Rosbury